Cell type annotation from marker genes
In single-cell, we have no prior information of which cell type each cell belongs. To assign cell type labels, we first project all cells in a shared embedded space, then we find communities of cells that show a similar transcription profile and finally we check what cell type specific markers are expressed. If more than one marker gene is available, statistical methods can be used to test if a set of markers is enriched in a given cell population.
In this notebook we showcase how to use decoupler for cell type annotation with the 3k PBMCs 10X data-set. The data consists of 3k PBMCs from a Healthy Donor and is freely available from 10x Genomics here from this webpage
Note
This tutorial assumes that you already know the basics of decoupler. Else, check out the Usage tutorial first.
Loading packages
First, we need to load the relevant packages, scanpy to handle scRNA-seq data and decoupler to use statistical methods.
[1]:
import scanpy as sc
import decoupler as dc
# Only needed for visualization:
import matplotlib.pyplot as plt
import seaborn as sns
Single-cell processing
Loading the data-set
We can download the data easily using scanpy:
[2]:
adata = sc.datasets.pbmc3k()
adata
[2]:
AnnData object with n_obs × n_vars = 2700 × 32738
var: 'gene_ids'
QC, projection and clustering
Here we follow the standard pre-processing steps as described in the scanpy vignette. These steps carry out the selection and filtration of cells based on quality control metrics, the data normalization and scaling, and the detection of highly variable features.
Note
This is just an example, these steps should change depending on the data.
[3]:
# Basic filtering
sc.pp.filter_cells(adata, min_genes=200)
sc.pp.filter_genes(adata, min_cells=3)
# Annotate the group of mitochondrial genes as 'mt'
adata.var['mt'] = adata.var_names.str.startswith('MT-')
sc.pp.calculate_qc_metrics(adata, qc_vars=['mt'], percent_top=None, log1p=False, inplace=True)
# Filter cells following standard QC criteria.
adata = adata[adata.obs.n_genes_by_counts < 2500, :]
adata = adata[adata.obs.pct_counts_mt < 5, :]
# Normalize the data
sc.pp.normalize_total(adata, target_sum=1e4)
sc.pp.log1p(adata)
# Identify the 2000 most highly variable genes
sc.pp.highly_variable_genes(adata, min_mean=0.0125, max_mean=3, min_disp=0.5)
# Filter higly variable genes
adata.raw = adata
adata = adata[:, adata.var.highly_variable]
# Regress and scale the data
sc.pp.regress_out(adata, ['total_counts', 'pct_counts_mt'])
sc.pp.scale(adata, max_value=10)
/home/badi/miniconda3/envs/decoupler/lib/python3.8/site-packages/scanpy/preprocessing/_normalization.py:155: UserWarning: Revieved a view of an AnnData. Making a copy.
view_to_actual(adata)
Then we group cells based on the similarity of their transcription profiles. To visualize the communities we perform UMAP reduction.
[4]:
# Generate PCA features
sc.tl.pca(adata, svd_solver='arpack')
# Compute distances in the PCA space, and find cell neighbors
sc.pp.neighbors(adata, n_neighbors=10, n_pcs=40)
# Generate UMAP features
sc.tl.umap(adata)
# Run leiden clustering algorithm
sc.tl.leiden(adata)
# Visualize
sc.pl.umap(adata, color='leiden', title='RNA UMAP',
frameon=False, legend_fontweight='normal', legend_fontsize=15)
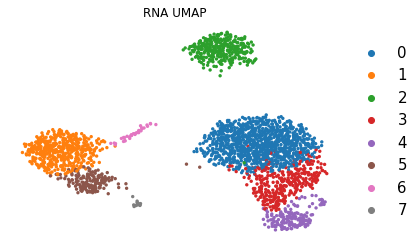
At this stage, we have identified communities of cells that show a similar transcriptomic profile, and we would like to know to which cell type they probably belong.
Marker genes
To annotate single cell clusters, one can use cell type specific marker genes. These are genes that are mainly expressed exclusively by a specific cell type, making them useful to distinguish heterogeneous groups of cells. Marker genes were discovered and annotated in previous studies and there are some resources that collect and curate them.
Omnipath is one of the largest available databases of curated prior knowledge. Among its resources, there is PanglaoDB, a database of cell type markers, which can be easily accessed using a wrapper to Omnipath from decoupler.
Note
If you encounter bugs with Omnipath, sometimes is good to just remove its cache using: rm $HOME/.cache/omnipathdb/*
[5]:
# Query Omnipath and get PanglaoDB
markers = dc.get_resource('PanglaoDB')
markers
[5]:
| genesymbol | canonical_marker | cell_type | germ_layer | human | human_sensitivity | human_specificity | mouse | mouse_sensitivity | mouse_specificity | ncbi_tax_id | organ | ubiquitiousness | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | CTRB1 | False | Enterocytes | Endoderm | True | 0.0 | 0.00439422 | True | 0.00331126 | 0.0204803 | 9606 | GI tract | 0.017 |
| 1 | CTRB1 | True | Acinar cells | Endoderm | True | 1.0 | 0.000628931 | True | 0.957143 | 0.0159201 | 9606 | Pancreas | 0.017 |
| 2 | KLK1 | True | Acinar cells | Endoderm | True | 0.833333 | 0.00503145 | True | 0.314286 | 0.0128263 | 9606 | Pancreas | 0.013 |
| 3 | KLK1 | False | Goblet cells | Endoderm | True | 0.588235 | 0.00503937 | True | 0.903226 | 0.0124084 | 9606 | GI tract | 0.013 |
| 4 | KLK1 | False | Epithelial cells | Mesoderm | True | 0.0 | 0.00823306 | True | 0.225806 | 0.0137585 | 9606 | Epithelium | 0.013 |
| ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... |
| 8472 | SLC14A1 | True | Urothelial cells | Mesoderm | True | 0.0 | 0.0181704 | True | 0.0 | 0.0 | 9606 | Urinary bladder | 0.008 |
| 8473 | UPK3A | True | Urothelial cells | Mesoderm | True | 0.0 | 0.0 | True | 0.0 | 0.0 | 9606 | Urinary bladder | 0.0 |
| 8474 | UPK1A | True | Urothelial cells | Mesoderm | True | 0.0 | 0.0 | True | 0.0 | 0.0 | 9606 | Urinary bladder | 0.0 |
| 8475 | UPK2 | True | Urothelial cells | Mesoderm | True | 0.0 | 0.0 | True | 0.0 | 0.0 | 9606 | Urinary bladder | 0.0 |
| 8476 | UPK3B | True | Urothelial cells | Mesoderm | True | 0.0 | 0.0 | True | 0.0 | 0.0 | 9606 | Urinary bladder | 0.0 |
8477 rows × 13 columns
Since our data-set is from human cells, and we want best quality of the markers, we can filter by canonical_marker and human:
[6]:
# Filter by canonical_marker and human
markers = markers[(markers['human']=='True')&(markers['canonical_marker']=='True')]
# Remove duplicated entries
markers = markers[~markers.duplicated(['cell_type', 'genesymbol'])]
markers
[6]:
| genesymbol | canonical_marker | cell_type | germ_layer | human | human_sensitivity | human_specificity | mouse | mouse_sensitivity | mouse_specificity | ncbi_tax_id | organ | ubiquitiousness | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CTRB1 | True | Acinar cells | Endoderm | True | 1.0 | 0.000628931 | True | 0.957143 | 0.0159201 | 9606 | Pancreas | 0.017 |
| 2 | KLK1 | True | Acinar cells | Endoderm | True | 0.833333 | 0.00503145 | True | 0.314286 | 0.0128263 | 9606 | Pancreas | 0.013 |
| 5 | KLK1 | True | Principal cells | Mesoderm | True | 0.0 | 0.00814536 | True | 0.285714 | 0.0140583 | 9606 | Kidney | 0.013 |
| 7 | KLK1 | True | Plasmacytoid dendritic cells | Mesoderm | True | 0.0 | 0.00820189 | True | 1.0 | 0.0129136 | 9606 | Immune system | 0.013 |
| 8 | KLK1 | True | Endothelial cells | Mesoderm | True | 0.0 | 0.00841969 | True | 0.0 | 0.0149153 | 9606 | Vasculature | 0.013 |
| ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... |
| 8472 | SLC14A1 | True | Urothelial cells | Mesoderm | True | 0.0 | 0.0181704 | True | 0.0 | 0.0 | 9606 | Urinary bladder | 0.008 |
| 8473 | UPK3A | True | Urothelial cells | Mesoderm | True | 0.0 | 0.0 | True | 0.0 | 0.0 | 9606 | Urinary bladder | 0.0 |
| 8474 | UPK1A | True | Urothelial cells | Mesoderm | True | 0.0 | 0.0 | True | 0.0 | 0.0 | 9606 | Urinary bladder | 0.0 |
| 8475 | UPK2 | True | Urothelial cells | Mesoderm | True | 0.0 | 0.0 | True | 0.0 | 0.0 | 9606 | Urinary bladder | 0.0 |
| 8476 | UPK3B | True | Urothelial cells | Mesoderm | True | 0.0 | 0.0 | True | 0.0 | 0.0 | 9606 | Urinary bladder | 0.0 |
5180 rows × 13 columns
For this example we will use these markers, but any collection of genes could be used. To see the list of available resources inside Omnipath, run dc.show_resources()
Enrichment with Over Representation Analysis
To test if a collection of genes are enriched in a given cell type, we will run Over Representation Analysis (ora), also known as Fisher exact test, but we could do it with any of the other available methods in decoupler.
ora selects the top 5% expressed genes for each cell, and tests if a collection of marker genes are enriched in the top expressed collection.
To run decoupler methods, we need an input matrix (mat), an input prior knowledge network/resource (net), and the name of the columns of net that we want to use.
[7]:
dc.run_ora(mat=adata, net=markers, source='cell_type', target='genesymbol', min_n=3, verbose=True)
1 features of mat are empty, they will be removed.
Running ora on mat with 2638 samples and 13713 targets for 114 sources.
100%|███████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████| 2638/2638 [00:03<00:00, 862.79it/s]
The obtained scores (-log10(p-value))(ora_estimate) and p-values (ora_pvals) are stored in the .obsm key:
[8]:
adata.obsm['ora_estimate']
[8]:
| source | Acinar cells | Adipocytes | Airway goblet cells | Alpha cells | Alveolar macrophages | Astrocytes | B cells | B cells memory | B cells naive | Basophils | ... | T cells | T follicular helper cells | T helper cells | T regulatory cells | Tanycytes | Taste receptor cells | Thymocytes | Trophoblast cells | Tuft cells | Urothelial cells |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AAACATACAACCAC-1 | 0.496369 | 0.093412 | -0.000000 | 1.443226 | 0.885021 | 0.581353 | 4.624325 | 1.459548 | 1.399835 | -0.000000 | ... | 13.523759 | -0.0 | 1.036748 | -0.000000 | -0.000000 | -0.0 | 3.325696 | -0.000000 | 1.036748 | -0.0 |
| AAACATTGAGCTAC-1 | 0.496369 | 1.114303 | -0.000000 | 0.569257 | -0.000000 | 1.119542 | 5.592250 | 8.719924 | 15.198236 | 0.306499 | ... | 2.431181 | -0.0 | 0.389675 | -0.000000 | -0.000000 | -0.0 | 0.827013 | 0.885021 | 2.858756 | -0.0 |
| AAACATTGATCAGC-1 | 0.496369 | 0.316389 | -0.000000 | 1.443226 | 0.885021 | 1.119542 | 0.903197 | 0.966789 | 2.580070 | 1.087856 | ... | 8.800720 | -0.0 | 1.036748 | -0.000000 | 0.663965 | -0.0 | 2.367740 | -0.000000 | 1.871866 | -0.0 |
| AAACCGTGCTTCCG-1 | 1.278757 | 1.659624 | -0.000000 | 1.443226 | 0.885021 | 0.195977 | 0.903197 | 0.074112 | 0.923023 | 0.306499 | ... | 0.718715 | -0.0 | -0.000000 | -0.000000 | -0.000000 | -0.0 | 0.298770 | -0.000000 | 0.389675 | -0.0 |
| AAACCGTGTATGCG-1 | -0.000000 | -0.000000 | 0.885021 | 0.569257 | 0.885021 | -0.000000 | 0.903197 | 0.074112 | 0.246287 | 1.623875 | ... | 3.162941 | -0.0 | 1.036748 | -0.000000 | 0.663965 | -0.0 | 0.298770 | -0.000000 | 0.389675 | -0.0 |
| ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... |
| TTTCGAACTCTCAT-1 | 1.278757 | 0.661862 | -0.000000 | 1.443226 | 0.885021 | 0.581353 | 1.469558 | 0.262017 | 1.399835 | 1.087856 | ... | 1.198738 | -0.0 | 0.389675 | 0.569257 | 0.663965 | -0.0 | 2.367740 | -0.000000 | 1.036748 | -0.0 |
| TTTCTACTGAGGCA-1 | -0.000000 | -0.000000 | -0.000000 | 1.443226 | 0.885021 | 0.195977 | 2.135997 | 1.459548 | 1.954926 | 1.087856 | ... | 1.198738 | -0.0 | -0.000000 | 0.569257 | 0.663965 | -0.0 | 0.827013 | -0.000000 | -0.000000 | -0.0 |
| TTTCTACTTCCTCG-1 | -0.000000 | -0.000000 | 0.885021 | 1.443226 | -0.000000 | 0.195977 | 10.042270 | 10.852223 | 15.198236 | 0.089844 | ... | 1.773449 | -0.0 | 0.389675 | -0.000000 | -0.000000 | -0.0 | 3.325696 | -0.000000 | 1.036748 | -0.0 |
| TTTGCATGAGAGGC-1 | -0.000000 | 0.316389 | 0.885021 | 0.569257 | -0.000000 | 0.195977 | 6.621109 | 7.714603 | 12.817177 | 0.089844 | ... | 1.198738 | -0.0 | 0.389675 | 0.569257 | -0.000000 | -0.0 | 1.527899 | -0.000000 | 1.036748 | -0.0 |
| TTTGCATGCCTCAC-1 | 0.496369 | 0.093412 | -0.000000 | -0.000000 | 0.885021 | -0.000000 | 0.454158 | 0.966789 | 1.399835 | 0.306499 | ... | 7.731358 | -0.0 | 1.036748 | -0.000000 | 1.657599 | -0.0 | 1.527899 | -0.000000 | 1.871866 | -0.0 |
2638 rows × 114 columns
Visualization
To visualize the obtianed scores, we can re-use many of scanpy’s plotting functions. First though, we need to extract them from the adata object.
[9]:
acts = dc.get_acts(adata, obsm_key='ora_estimate')
acts
[9]:
AnnData object with n_obs × n_vars = 2638 × 114
obs: 'n_genes', 'n_genes_by_counts', 'total_counts', 'total_counts_mt', 'pct_counts_mt', 'leiden'
uns: 'log1p', 'hvg', 'pca', 'neighbors', 'umap', 'leiden', 'leiden_colors'
obsm: 'X_pca', 'X_umap', 'ora_estimate', 'ora_pvals'
dc.get_acts returns a new AnnData object which holds the obtained activities in its .X attribute, allowing us to re-use many scanpy functions, for example:
[10]:
sc.pl.umap(acts, color='NK cells')
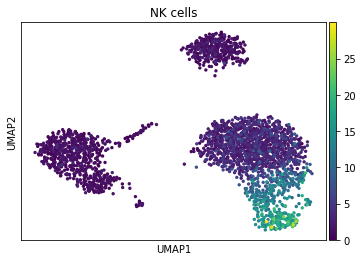
The cells highlighted seem to be enriched by NK cell marker genes.
Annotation
With decoupler we can also see what is the mean enrichment per group, in our case the cluster groups obtained from clustering:
[11]:
mean_enr = dc.summarize_acts(acts, groupby='leiden', min_std=1)
mean_enr
[11]:
| B cells | B cells memory | B cells naive | Dendritic cells | Gamma delta T cells | Macrophages | Megakaryocytes | Microglia | Monocytes | NK cells | Neutrophils | Plasma cells | Platelets | T cells | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1.674612 | 1.206118 | 2.126226 | 3.089706 | 1.282204 | 0.656916 | 0.558249 | 0.824813 | 1.969143 | 2.502024 | 0.733604 | 1.098518 | 2.097416 | 8.560976 |
| 1 | 1.491951 | 0.537041 | 1.358659 | 12.386445 | 0.392409 | 5.205774 | 0.994419 | 3.438604 | 6.913848 | 0.876738 | 4.510537 | 1.040281 | 2.978631 | 1.319387 |
| 2 | 6.927173 | 7.202431 | 12.459538 | 7.860511 | 0.424341 | 0.726596 | 0.453553 | 1.126456 | 1.839738 | 1.016634 | 0.781631 | 4.108602 | 2.022158 | 2.391670 |
| 3 | 1.528638 | 1.003478 | 1.688443 | 3.882292 | 5.015036 | 1.082678 | 0.747354 | 1.013499 | 1.990285 | 8.912252 | 0.738189 | 1.171692 | 2.690642 | 10.510677 |
| 4 | 1.058014 | 0.690444 | 1.214375 | 3.754120 | 12.026393 | 1.798656 | 1.006925 | 1.552309 | 2.541472 | 16.074585 | 1.316749 | 1.296880 | 2.992357 | 6.312927 |
| 5 | 2.046917 | 0.715149 | 1.472404 | 12.163185 | 0.667618 | 5.303366 | 0.701650 | 3.465839 | 6.493895 | 0.895001 | 2.756551 | 0.970993 | 3.152135 | 1.381764 |
| 6 | 1.753542 | 0.827478 | 2.325148 | 13.919129 | 0.783363 | 3.639544 | 0.616080 | 2.822582 | 4.251034 | 0.765348 | 2.401234 | 1.879233 | 2.796829 | 1.497420 |
| 7 | 0.801249 | 0.592690 | 1.186940 | 3.111120 | 0.701021 | 1.905558 | 5.641654 | 1.086733 | 0.595006 | 0.993758 | 1.055535 | 1.049253 | 23.893217 | 1.044401 |
We can visualize which cell types are more enriched for each cluster using seaborn:
[12]:
sns.clustermap(mean_enr, xticklabels=mean_enr.columns, cmap='viridis')
plt.show()
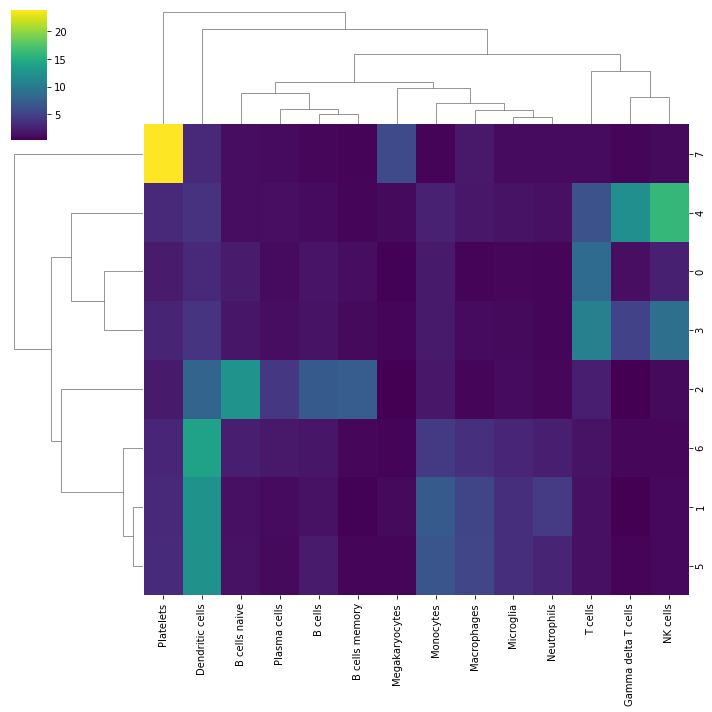
From this plot we see that cluster 7 belongs to Platelets, cluster 4 appear to be NK cells, custers 0 and 3 might be T-cells, cluster 2 should be some sort of B cells and that clusters 6,5 and 1 are Dendritic cells or Monocytes
We can also get what is the maximum value per group:
[13]:
annotation_dict = dc.assign_groups(mean_enr)
annotation_dict
[13]:
{'0': 'T cells',
'1': 'Dendritic cells',
'2': 'B cells naive',
'3': 'T cells',
'4': 'NK cells',
'5': 'Dendritic cells',
'6': 'Dendritic cells',
'7': 'Platelets'}
Which we can use to annotate our data-set:
[14]:
# Add cell type column based on annotation
adata.obs['cell_type'] = [annotation_dict[clust] for clust in adata.obs['leiden']]
# Visualize
sc.pl.umap(adata, color='cell_type')
/home/badi/miniconda3/envs/decoupler/lib/python3.8/site-packages/anndata/_core/anndata.py:1220: FutureWarning: The `inplace` parameter in pandas.Categorical.reorder_categories is deprecated and will be removed in a future version. Reordering categories will always return a new Categorical object.
c.reorder_categories(natsorted(c.categories), inplace=True)
... storing 'cell_type' as categorical
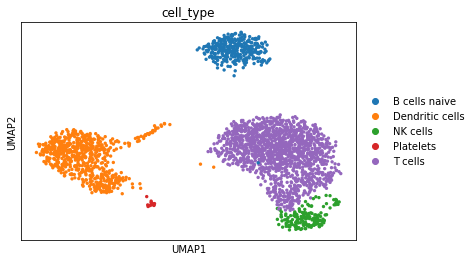
Compared to the annotation obtained by the scanpy tutorial, it is very simmilar.

Note
Cell annotation should always be revised by an expert in the tissue of interest, this notebook only shows how to generate a first draft of it.